|
On November 22, 2019, Zhou Qilin and Zhu
Shoufei's team, Nankai University published an article entitled "Highly
enantioselective carbene insertion into N–H bonds of aliphatic amines"
online in Science, which reported highly enantioselective carbene insertion
into N–H bonds of aliphatic amines using two catalysts in tandem. This research
not only solves the long-term challenges of enantioselective carbene insertion
reactions, but also provides a potential general strategy for transition
metal-catalyzed asymmetric transformations involving strong coordination
substrates. Eric N. Jacobsen from Harvard published a review article entitled
"A catalytic one-two punch" in the Science with the same issue number, which systematically
interpreted the research and gave it a high evaluation.
First Author: Li Maolin
Corresponding author: Zhou Qilin
Communication unit: Nankai University
Aliphatic amines strongly coordinate, and
therefore easily inhibit, the activity of transition-metal catalysts, posing a
marked challenge to nitrogen-hydrogen (N–H) insertion reactions. Here, we
report highly enantioselective carbene insertion into N–H bonds of aliphatic
amines using two catalysts in tandem: an achiral copper complex and chiral
amino-thiourea. Coordination by a homoscorpionate ligand protects the copper
center that activates the carbene precursor. The chiral amino-thiourea catalyst
then promotes enantioselective proton transfer to generate the stereocenter of
the insertion product. This reaction couples a wide variety of diazo esters and
amines to produce chiral α-alkyl α–amino acid derivatives.
Chiral amines are ubiquitous in natural
products, pharmaceuticals, and agrochemicals. Approximately 43% of the top 200
prescription medicines in 2016 contain an aliphatic amine moiety (Fig. 1A). The
development of highly enantioselective transition-metal–catalyzed reactions
that form C–N bonds is thus of long-standing interest in synthetic chemistry.
Transition-metal–catalyzed carbenoid insertion into N–H bonds has proven a
straightforward method in this respect, benefitting from mild reaction
conditions, good functional group tolerance, and readily available reactants.
Recently, chiral transition-metal catalysts have been successfully applied to
enantioselective N–H insertion reactions in the synthesis of natural or
unnatural chiral α–amino acid derivatives. However, these reactions have been
restricted to aromatic amines or amides
(Fig. 1B). Aliphatic amines are comparatively stronger Lewis bases and
thus poison the metal catalysts by strong coordination, interfering with
generation of the metal carbenoid. Moreover, excess aliphatic amines can
displace the ylide from metal-ylide intermediates, leading to racemic product
formation from the free ylide (Fig. 1C, upper).
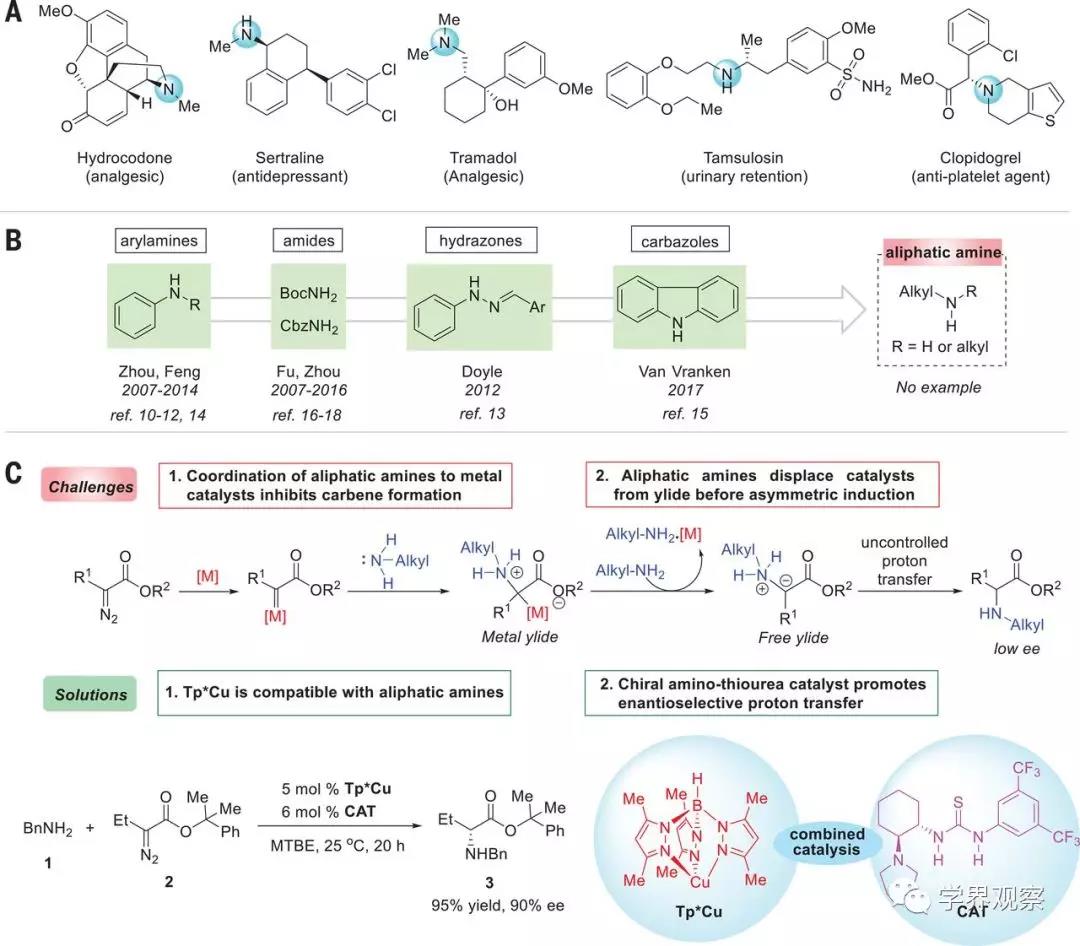
Fig. 1 Strategy for enantiocontrol of N–H
insertion reactions of aliphatic amines with carbenes.
(A) Representative drugs demonstrating the
ubiquity of chiral aliphatic amines in bioactive molecules. (B) Amine sources
reported for enantioselective N–H insertion reactions. (C) Enantioselective
transition-metal–catalyzed N–H insertion reactions with aliphatic amines:
challenges and solutions. Optimal reaction conditions: The reaction of 1 (0.2
mmol), 2 (0.22 mmol), Tp*Cu (5 mole %), and CAT (6 mol %) was carried out in 3
ml of methyl tert-butyl ether (MTBE) at 25°C for 20 hours. BnNH2, benzylamine;
BocNH2, tert-butyl carbamate; CbzNH2, benzyl carbamate; Me, methyl; Et, ethyl;
Ph, phenyl; M, metal; ref., reference.
Academician Zhou Qilin and Professor Zhu
Shoufei from Nankai University envisioned that a combination of two catalysts
might address these challenges: An achiral transition-metal catalyst compatible
with aliphatic amines would generate the ylide intermediate, and a separate
chiral catalyst would then promote enantioselective proton transfer. After
exploring various transition-metal catalysts and chiral H-bonding catalysts in
the N–H insertion reaction of α-diazobutanoates with benzylamine (tables S1 to
S5), the authors report here the success of this approach, pairing the
homoscorpionate-coordinated copper complex Tp*Cu [Tp* is
hydrotris(3,5-dimethylpyrazolyl)borate] with a chiral amino-thiourea (CAT)
bearing a pyrrolidine motif (Fig. 1C, lower). The reaction provides efficient,
highly enantioselective access to chiral α-alkyl α–amino acid derivatives
bearing secondary and tertiary amino substituents, which are difficult to
prepare by other methods.
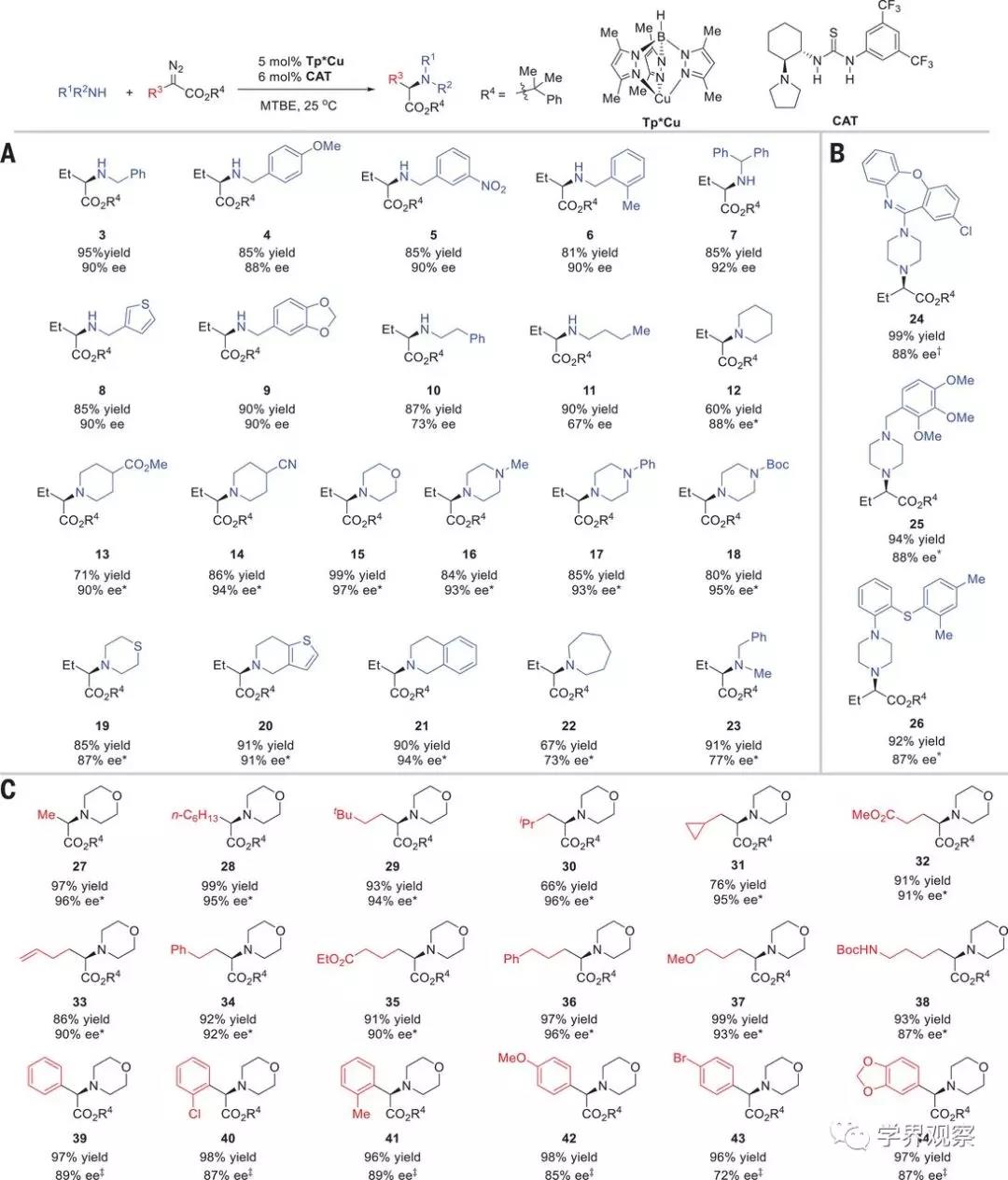
Fig. 2 Scope of aliphatic amines and
α-diazo esters in the enantioselective N–H insertion reaction.
Reaction conditions: amines (0.2 mmol),
α-diazo esters (0.22 mmol), Tp*Cu (5 mol %), CAT (6 mol %), 3 ml MTBE, 25°C, 20
hours. Isolated yields are given. The ee values were determined by
high-performance liquid chromatography. (A) Scope of aliphatic amines. (B)
Application to enantioselective late-stage functionalization of
pharmaceuticals. (C) Scope of α-diazo esters. *Diazo esters (0.3 mmol), 36
hours. †Diazo esters (0.3 mmol), MTBE:CH2Cl2 = 10:1, 36 hours. ‡Diazo esters
(0.3 mmol), 40°C, 20 hours. tBu, tert-butyl; iPr, iso-propyl.
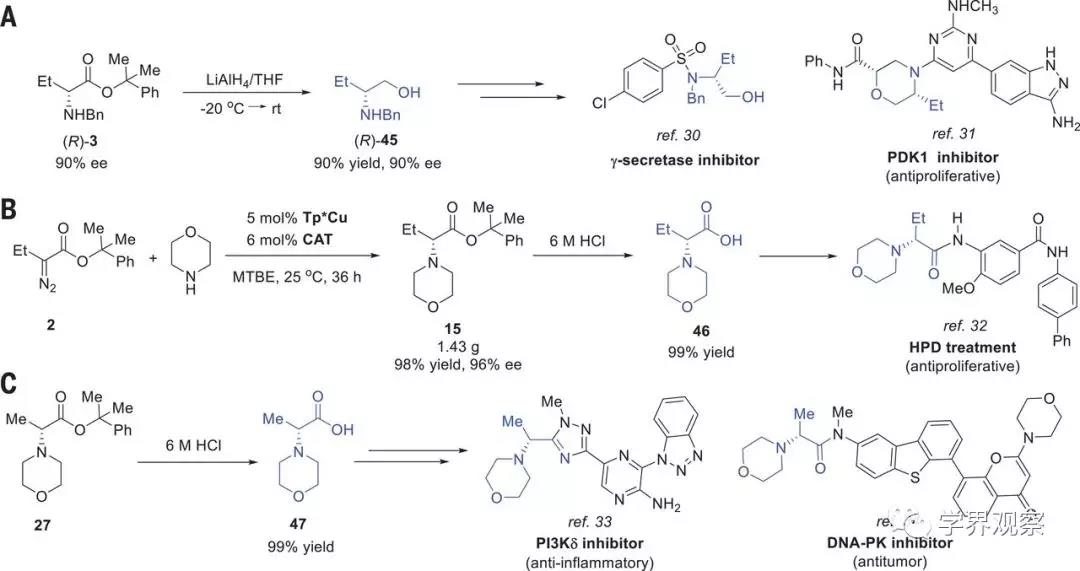
Fig. 3 Synthetic transformations of the N−H
insertion products.
(A) Transformation of 3 to
(R)-2-benzylamino-butanol [(R)-45], a key intermediate for the synthesis of
bioactive molecules. THF, tetrahydrofuran; rt, room temperature. (B) Formal
synthesis of HPD treatment agents with the N−H insertion as key step. (C)
Transformation of 27 to 47, a key intermediate for the synthesis of bioactive
molecules.
To gain a deeper understanding of the
mechanism of the NH insertion reaction, the authors performed kinetic analysis
using online infrared (IR) spectroscopy. In a nutshell, the kinetic, NMR, and
UV studies are consistent with the Tp*Cu•CAT complex, rather than the
Tp*Cu•BnNH2 complex, as the resting state of the catalyst in the reaction.
Although the Tp*Cu•CAT complex is the main resting state of the copper, free
Tp*Cu is still evident under the reaction conditions (fig. S10) and can react
with the diazo compound.

Fig. 4 Mechanistic studies.
(A) Kinetic profiles of Cu-catalyzed N−H insertion reaction
of 2 and BnNH2. (B) Calculated Gibbs free energy (ΔG) of Cu-ylide, free ylide,
and free enol. Lowest-energy ground-state structure of the Tp*Cu•CAT complex.
Structures of alternative higher-energy complexes are provided in fig. S20. (C)
DFT-optimized lowest-energy transition structures for R and S products.
Calculations were performed at the m062x-D3/def2tzvpp//m062x-D3/def2svp level.
Structures of alternative higher-energy complexes are provided in figs. S21 to
S25. (D) Influence of different Tp ligands. (E) Proposed catalytic cycle for
the enantioselective carbene insertion into N–H bonds of aliphatic amines.
conv., conversion; DMSO, dimethyl sulfoxide; equiv, equivalents.
Several other tris(pyrazolyl)borate (Tp)
ligands bearing different substituents on the pyrazol rings were also evaluated
under the standard reaction conditions (Fig. 4D). Despite a large fluctuation
in the yield, the in situ IR studies showed that all tested Tp ligands promoted
high conversions and that the major by-product was 2-phenylpropan-2-yl
but-2-enoate, resulting from the β-H migration of the metal carbenoid (figs.
S15 to S19). Modifying the Tp ligands also influenced the enantioselectivity
when the same chiral thiourea catalyst was used, indicating involvement of the
copper catalyst in the enantio-determining step. By contrast, upon tuning the
electronic properties of the arene ring of the chiral thiourea catalyst, the
enantioselectivity decreased precipitously, whereas the yield remained almost
unchanged (table S7). We again hypothesize that copper coordination enhances
the Brønsted acidity of the thiourea catalyst while minimally influencing the
distant site of enantioinduction (Fig. 4C).
On the basis of the aforementioned
mechanistic studies, a catalytic cycle is proposed in Fig. 4E. The Tp*Cu•CAT
complex serves as a resting state of the catalyst and dissociates to release
Tp*Cu, which catalyzes transformation of the diazo ester into the metal
carbenoid in the rate-determining step. Nucleophilic attack on the metal
carbenoid by the aliphatic amine generates a metal ylide. The catalyst CAT
displaces the ylide from the metal-ylide intermediate to generate free enol and
the Tp*Cu•CAT complex. The Tp*Cu•CAT complex then promotes proton transfer in
the free enol through a push-pull mechanism: The amino moiety accepts a proton
from the hydroxy group of the enol while the thiourea moiety donates a proton
to the β-carbon of the enol.
The author states that the success of the
overall conversion depends on the comprehensive performance of achiral copper
catalysts and chiral organic catalysts. This study not only addresses the
long-term challenges of enantioselective carbene insertion reactions, but also
provides a potential general strategy for transition metal-catalyzed asymmetric
transformations involving strong coordination substrates.
The author states that the success of the
overall transformation relies on the combined properties of the achiral copper
catalyst and chiral organocatalyst. This study not only solves a long-standing
challenge in enantioselective carbene insertion reactions but also provides a
potentially general strategy for transition-metal–catalyzed asymmetric
transformations involving strongly coordinating substrates.
Harvard University Jacobsen commented on
the research during the same period of Science, stating that "The
cooperative action of achiral transition metal complexes with chiral
hydrogen-bond donors holds enormous potential for achieving new asymmetric
transformations. Organotransition-metal chemistry provides access to a wealth
of reactivity modes inaccessible to organocatalysts, and chiral
hydrogen-bond-donor catalysts have been found to promote enantiocontrol through
a rich variety of noncovalent mechanisms. The system developed by Li et al.
represents a compelling glimpse into some of the possibilities."

Academician Zhou Qilin, organic chemist, professor
of Nankai University. Born in Nanjing, Jiangsu in February 1957. He graduated
from the Department of Chemistry of Lanzhou University in July 1982, and
obtained his master's and doctoral degrees from Shanghai Institute of Organic
Chemistry, Chinese Academy of Sciences in 1985 and 1987, respectively. He has
performed postdoctoral research at Max-Planck Polymer Research Institute in
Germany, Basel University in Switzerland, and Trinity University in the United
States. In 2009 he was elected an academician of the Chinese Academy of
Sciences. Mainly engaged in asymmetric catalytic synthesis. It involves the
structural design of chiral catalysts, catalyst synthesis, corresponding
asymmetric synthesis reactions and the application of these chiral catalysts to
the synthesis of chiral molecules. A new class of chiral spiro ligands was
developed, and a series of new chiral spiro catalysts were designed and
synthesized based on this kind of ligands. These catalysts show excellent
catalytic activity and enantioselectivity in a series of asymmetric synthesis
reactions such as asymmetric catalytic hydrogenation, carbon-carbon bond and
carbon-heteroatom bond formation. The research results have been applied in the
synthesis of hands and things.

Professor Zhu Shoufei, received a Bachelor
of Science degree and a Doctor of Science degree from the School of Chemistry,
Nankai University in 2000 and 2005, respectively, and a postdoctoral fellow at
the University of Tokyo, Japan from 2012 to 2013. He has worked in the School
of Chemistry, Nankai University from 2005 to present, and received the National
Natural Science Fund in 2016 Funded by the Outstanding Youth Fund. He has long
been engaged in the research of catalytic organic synthetic chemistry, focusing
on several types of important organic synthesis reactions that take hydrogen
transfer as the key step, and proposed the concept of "chiral proton
shuttle", which provides a new solution for metal-catalyzed asymmetric
proton transfer reactions. ; Discovered the catalytic carbene insertion
reaction of boron-hydrogen bonds, provided a new method for the synthesis of
organoboron compounds; developed a variety of efficient catalysts for
chlorination and hydrosilylation of olefins, and realized a variety of
important biologically active molecules Efficient synthesis. He has published
more than 100 research papers and authored two chapters: 5 Chinese patents have
been authorized. He has won 2 first prizes in Tianjin Natural Science (both 3rd
person), Youth Chemistry Award of Chinese Chemical Society, Youth Chiral
Chemistry Award of Chinese Chemical Society, Tianjin Youth Science and
Technology Award, Tianjin Youth May Fourth Medal, Asia Core Program Lectureship
Award and other awards.

First author Mao-Lin Li of Nankai
University
References:
https://science.sciencemag.org/content/366/6468/990
https://science.sciencemag.org/content/366/6468/948
|


 Position:
Home
>>
News
>>
正文
Position:
Home
>>
News
>>
正文

