Glycosylation is an important means of modifying bioactive molecules in nature, and many natural products with important physiological activities have glycosylation modifications. In addition to the common O-glycosides and N-glycosides, C-glycoside structures are also widely present in natural products with biological activity. Since the C-glycoside structure has higher stability than O-glycoside and N-glycoside, and is not easily degraded by hydrolytic enzymes in vivo, C-glycosidation transformation has become an effective strategy for structural optimization of glycoconjugates. For example, in recent years, the oral hypoglycemic drugs Dapagliflozin and Sotagliflozin have been gradually evolved from the leading compound of O-glycoside, Phlorizin. According to the different positions where the carbon skeleton is connected to the sugar unit, C-glycosides can be divided into classic C-glycosides (the carbon skeleton is connected to the C1 position of the sugar unit, such as dapagliflozin) and non-classical C-glycosides (the carbon skeleton and The C4 position of furanose or the C5 position of pyranose are connected, such as Sotagliflozin, Scheme 1). The research on the method suitable for non-classical C-glycoside synthesis is relatively rare compared with the classical C-glycoside-rich synthesis method.

Scheme1 Classical vs non-classical aryl C-glycosides
Recently, Professor Chen gong’s group, Nankai University reported a radical-mediated method for diastereoselective synthesis of non-classical heteroaryl C-glycosides via Miniscitype alkylation of N-heteroarenes with 4-glycosyl-1,4-dihydropyridine (DHP) reagents. These DHP reagents serve as convenient precursors for various glycosyl radicals under the activation of single electron transfer (SET) oxidation by persulfate and visible light irradiation with or without photocatalyst. Notably, the reaction showed excellent stereoselectivity, and the absolute configuration of the product was verified by a single crystal structure
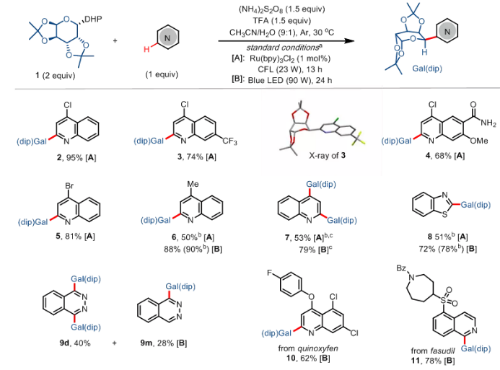
a) Isolated yields on 0.2 mmol scale. Excellent stereoselectivities (dr > 20:1) were observed. b) NMR yield. c) 4 equivalent of 1 was used.
Scheme 2 the expansion of Heterocyclic substrate
In addition to galactose, this method is also suitable for the reaction of furanose substrates with nitrogen heterocyclic compounds, providing a new strategy for the diversified synthesis of carbon-nucleoside molecules (Scheme 3). A pair of diastereomers of nucleoside derivative 12 can react with tetrachloroquinoline under standard conditions to obtain the target products 13 and 14 with similar stereoselectivity. 4-xylose-dihydropyridine containing different protecting groups can be medium and above The yield and stereoselectivity of the glycosylation product were synthesized, and the stereo configuration of product 20 was also verified by single crystal diffraction
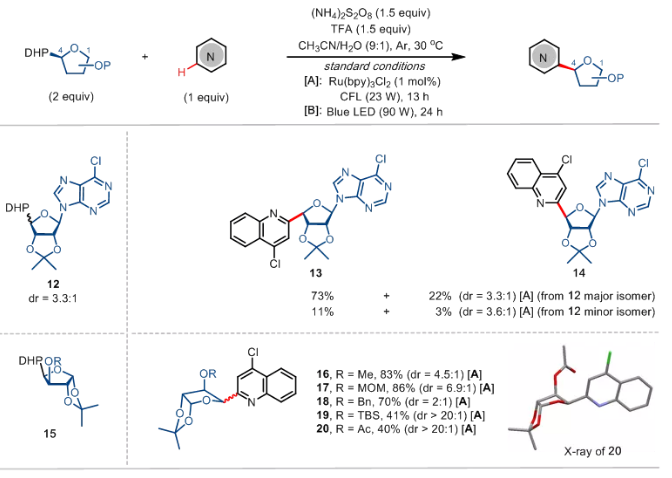
Scheme 3 the expansion of furanose substrate
Finally, the author speculates on the mechanism of the reaction and believes that the reaction has gone through the classic Minisci reaction process, that is, the glycosyl radical species G(II) under acidic conditions adds to the protonated nitrogen heterocycle to generate intermediate III. , and then aromatize under oxidizing conditions to produce the final product IV (Scheme 4).
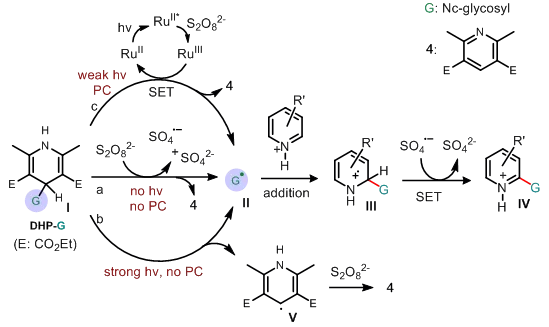
Scheme 4 Reaction mechanism
Related results were recently reported in Science China Chemistry. See the details: Qingbing Wang, Juan Duan, Pingping Tang, Gong Chen* & Gang He*. Synthesis of Non-classical Heteroaryl C-Glycosides via Minisci-type Alkylation of N-Heteroarenes with 4-Glycosyl-dihydropyridines. Sci. China Chem. 2020, doi: 10.1007/s11426-020-9813-5.


 Position:
Home
>>
News
>>
正文
Position:
Home
>>
News
>>
正文

